Circular RNA
Circular RNA
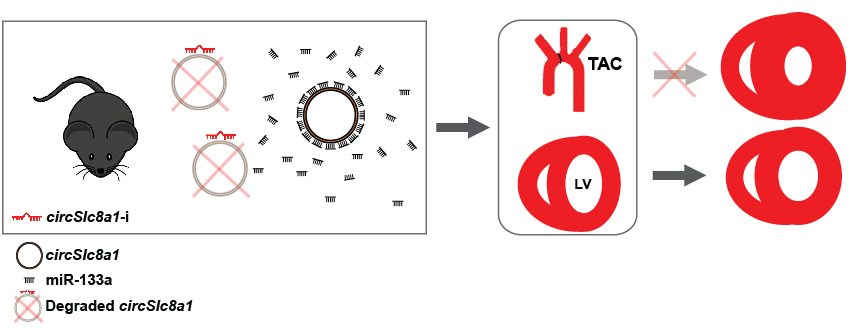
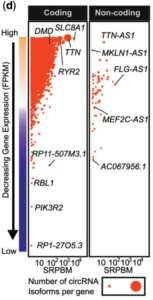
Circular RNAs (circRNAs) belong to a class of non-coding RNA. They are single-stranded RNA circularized as a result from a back-splicing event and lacks the traditional 5’cap and 3’ poly-A tail. We1 and others2 have previously described the expression landscape of circular RNA (circRNA) in mouse and human hearts. However, the functional relevance of many of these abundantly expressed cardiomyocyte circRNA remains to be fully explored. Among the most abundant circRNA, one stems from the sodium-calcium exchanger gene, Slc8a1, exon 2 locus. Because of its very high abundance in cardiomyocytes we investigated the possible role of circSlc8a1 in the heart.
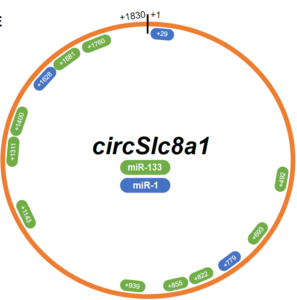
We performed a de novo miRNA screen using an array of 752 miRNAs with RNA recovered from a pull-down of endogenous cardiomyocyte circSlc8a1. MicroRNA-133a (miR-133a), with a prior well-recognized role in cardiac hypertrophy, was highly enriched in the fraction of circSlc8a1 pull-down (adjusted p-value < 0.001). We therefore followed-up validation of the functional interaction between circSlc8a1 and miR-133 using luciferase assays and reciprocal pull-down assays. In vivo, AAV9-mediated RNAi knockdown of circSlc8a1 attenuates cardiac hypertrophy from pressure-overload, whereas forced cardiomyocyte58 specific overexpression of circSlc8a1 resulted in heart failure. Molecular analyses showed targets of miR-133a including serum response factor (SRF), connective tissue growth factor (CTGF), adrenoceptor beta 1 (Adrb1) and adenylate cyclase 6 (Adcy6) to be regulated by circSlc8a1-directed intervention of knockdown and overexpression.
In summary, circSlc8a1 can function as an endogenous sponge for miR-133a in cardiomyocytes3. We propose that circSlc8a1 may serve as a novel therapeutic target for cardiac hypertrophy3.
1. Tan WL, Lim BT, Anene-Nzelu CG, Ackers-Johnson M, Dashi A, See K, Tiang Z, Lee DP, Chua WW, Luu TD, Li PY, Richards AM and Foo RS. A landscape of circular RNA expression in the human heart. Cardiovasc Res. 2017;113:298-309.
2. Werfel S, Nothjunge S, Schwarzmayr T, Strom TM, Meitinger T and Engelhardt S. Characterization of circular RNAs in human, mouse and rat hearts. J Mol Cell Cardiol. 2016;98:103-7.
3. Lim TB, Aliwarga E, Luu TDA, Li YP, Ng SL, Annadoray L, Sian S, Ackers-Johnson MA and Foo RS. Targeting the highly abundant circular RNA circSlc8a1 in cardiomyocytes attenuates pressure overload induced hypertrophy. Cardiovasc Res. 2019.
Tuan Danh Anh Luu, Yiqing Peter Li, Shi Ling Ng, Lavenniah Annadoray, Stephanie Sian, Matthew Andrew Ackers-Johnson, Roger Sik-Yin Foo

Add Your Comment